Atmospheric Lifetimes
The atmospheric lifetimes for the major HFCs, HFOs and HCFOs are in this table. Their emissions and atmospheric lifetimes dictate their atmospheric concentrations, which are monitored (see atmospheric concentrations).
HFOs and HCFOs have negligible contribution to global warming, they are not included in the basket of 6 greenhouse gases and are shortlived gases with lifetimes of days or months, which means they are removed from the atmosphere very quickly.

Widely used HFCs have lifetimes of years or decades. Greenhouse Gases (GHGs) that are much shorter lifetimes than CO2 such as methane – CH4 (lifetime ~12 years) and some HFCs such as HFC-32 (lifetime ~5.2 years) and HFC134a (lifetime ~13.4 years) behave very differently to long-lived CO2:
- The effect of these short-lifetime GHGs on global average temperature is much more closely controlled by their emissions rate as opposed to the cumulative total of emissions over time.
- Their relatively short-lifetimes mean that for a constant rate of emission, atmospheric concentrations of a short-lifetime gas quickly increase to the point at which the amount of the gas decaying out of the atmosphere each year is equal to the amount being added through new emissions, keeping atmospheric concentrations constant. This only causes a slow increase in global temperature as the deep oceans warm-up on the timescale of several centuries. Maintaining constant emissions of a short-lifetime gas would therefore consolidate the existing warming effect, rather than increasing it. This is unlike CO2, for which atmospheric concentrations and warming both continue to steadily increase with sustained emissions.
- Offsetting the slow additional increase in warming from short-lifetime gases would only require emissions of the short-lifetime gas to fall by less than 1% per year. Reducing the rate of emissions of a short-lifetime gas faster than around 1% year would in effect lead to lower atmospheric concentrations and a decrease in their effect on global warming.
Short-lifetime greenhouse gases affect the climate in qualitatively different ways to CO2, with constant rates of emission leading to an approximately constant level of raised global average temperature but not a sustained and continually increasing warming. Aggregation as ‘carbon dioxide equivalent’ fails to capture this fundamental difference in how emissions of short-lived and long-lived GHGs affect global temperature. However international comparability supports the continued use of existing ‘CO2 equivalence’ metrics (the 100 year GWP). From 2018: Framing and Context. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C page 66 “Climate forcers fall into two broad categories in terms of their impact on global temperature long-lived GHGs, such as CO2 and nitrous oxide (N2O), whose warming impact depends primarily on the total cumulative amount emitted over the past century or the entire industrial epoch; and short-lived climate forcers (SLCFs), such as methane and black carbon, whose warming impact depends primarily on current and recent annual emission. These different dependencies affect the emissions reductions required of individual forcers to limit warming to 1.5°C or any other level.”

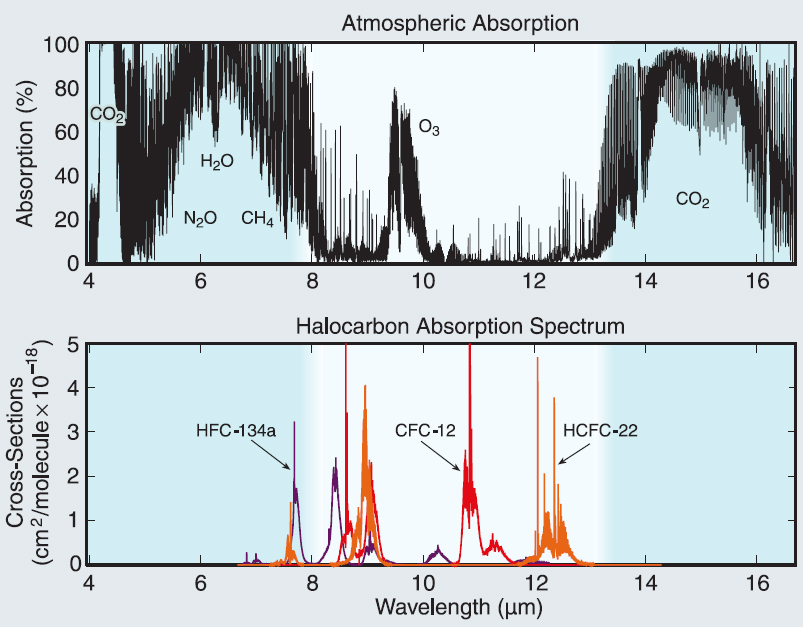
HFCs are greenhouse gases because they absorb Earth’s outgoing infrared radiation in a spectral range where energy is not removed by CO2 or water vapour. The carbon-fluorine bond absorbs infrared in this range. The magnitude of this effect depends on:
- the number of C-F bonds in the molecule
- its atmospheric lifetime which is determined by how rapidly it breaks down
- its concentration in the atmosphere, which is determined by emissions and atmospheric lifetime
Minimising the contribution to global warming can be achieved by reducing one or more of these factors, in particular by designing molecules that achieve the required technical and safety properties but with very short atmospheric lifetimes. However, retaining fluorine in a molecule has technical and safety benefits, for example it reduces flammability.
Why HFOs: Simple Chemistry
HFOs have C=C double bonds; the hydroxyl radical (OH) is the primary reactive agent in the atmosphere, reaction of OH radicals with C=C double bonds is very rapid; compared to a similar HFC the reaction of HFO is 2000 times faster.
Breakdown in the atmosphere
The hydroxyl radical (OH) is the primary reactive agent of the lower atmosphere and, in particular, it provides the dominant sink for HFCs and HFOs/HCFOs. Atmospheric lifetimes are determined primarily by reaction rates with hydroxyl radical. The local abundance of OH is mainly controlled by the local abundances of nitrogen oxides (NOx = NO + NO2), CO, CH4 and higher hydrocarbons, O3, and water vapour, as well as by the intensity of solar ultraviolet radiation.
Why do HFO and HCFOs breakdown rapidly?
The reaction of OH radicals with C=C double bonds is very rapid and dominates the atmospheric removal mechanism for HFOs and HCFOs. The inclusion of a double bond is a particularly effective method to increase the reactivity of organic molecules towards OH radicals.
Comparing the reactivity of HFC with HFO for reaction with OH radicals
CF3CF=CH2 (HFO-1234yf, GWP <1 AR5 value) is approximately 2000 times more reactive than CF3CF2CH3 (HFC-245cb, GWP 4620) [World Meteorological Organization, 2011. Scientific Assessment of Ozone Depletion: 2010, Global Ozone, Research and Monitoring Project – Report 5].