ANNEX to News item: Update on the PFAS restriction proposal and F-gases by the Norwegian Environment Agency
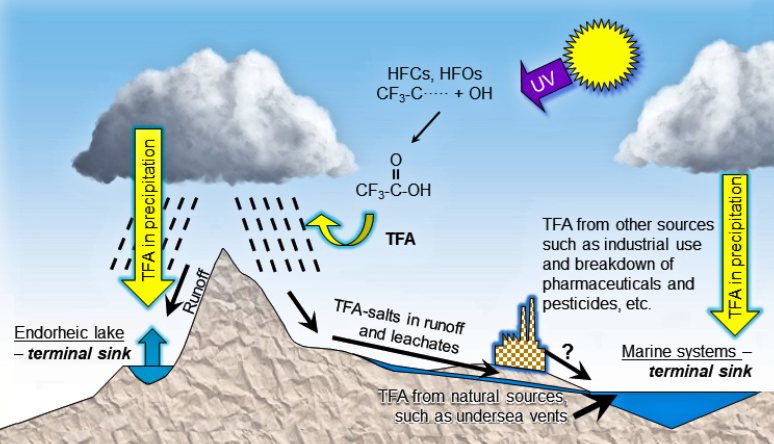
Image Reproduced from EEAP. 2019. Environmental Effects and Interactions of Stratospheric Ozone Depletion, UV Radiation, and Climate Change. 2018 Assessment Report. Nairobi: Environmental Effects Assessment Panel, United Nations Environment Programme (UNEP) 390 pp. https://ozone.unep.org/science/assessment/eeap
The Norwegian Environment Agency virtual briefing provided an update on the PFAS restriction proposal and F gases. This annex provides summaries of important conclusions from studies about TFA deposition from atmospheric degradation of HFO-1234yf due to the transition from HFC-134a as the virtual briefing did not discuss if this would result in any environmental effects. The virtual briefing is available at The universal PFAS restriction proposal and F-gases - Norwegian Environment Agency.
“Future emissions and atmospheric fate of HFC-1234yf from mobile air conditioners in Europe”[1]
These highest annual mean concentrations [of TFA] are at least 60 times lower than previously determined to be a safe level for the most sensitive aquatic life-forms. Rainwater concentrations during individual rain events would still be 1 order of magnitude lower than the no effect level.
“Ozone and TFA impacts in North America from degradation of 2,3,3,3-tetrafluoropropene (HFO-1234yf), a potential greenhouse gas replacement”[2].
Automobile air conditioning HFO-1234yf emissions are predicted to produce concentrations of TFA in Eastern U.S. rainfall at least double the values currently observed from all sources, natural and man-made. Our model predicts peak concentrations in rainfall of 1264 ng L-1, a level that is 80× lower than the lowest level considered safe for the most sensitive aquatic organisms.
“Estimated 2017 refrigerant emissions of 2, 3, 3, 3-tetrafluoropropene (HFC-1234yf) in the United States resulting from automobile air conditioning”[3]
This projected inventory is a necessary first step in analysing for potential atmospheric and ecosystem effects, such as ozone and trifluoroacetic acid production, that might result from widespread replacement of HFC-134a with HFC-1234yf.
“Deposition and rainwater concentrations of trifluoroacetic acid in the United States from the use of HFO-1234yf”[4]
In our simulation, the average TFA rainwater concentrations over the contiguous USA in May–September 2006 period and in each month remained below 100μgL−1 by 2 orders of magnitude. However, high TFA rainwater concentrations occurred during shorter time periods at locations with sparse precipitation. We found an average TFA rainwater concentration of 0.89μgL−1 for the contiguous USA in the May–September period, with mean values over this period peaking at 7.8μgL−1. These TFA rainwater concentrations are similar to rainwater concentrations found in previous modeling studies [Luecken et al., 2010; Henne et al., 2012], who used HFO-1234yf emissions lower by a factor of 3–6 compared to those in this work.
“Impacts of the Degradation of 2,3,3,3-Tetrafluoropropene into Trifluoroacetic Acid from Its Application in Automobile Air Conditioners in China, the United States, and Europe”[5]
The TFA concentrations in rainwater were strongly affected by the regional precipitation rates. North Africa and the Middle East regions with scant rainfall had extremely high TFA concentrations. The rainwater concentrations of TFA during individual rain events can exceed the level considered to be safe, indicating substantial potential regional risks from future HFO-1234yf use.
BUT see the 2021 paper for that investigates deposition for India, China, and the Middle East.
Trifluoroacetic acid deposition from emissions of HFO-1234yf in India, China, and the Middle East (2021)[6]:
The mean and the extremes of TFA rainwater concentrations calculated for the four emission scenarios from GEOS-Chem and WRF-Chem were orders of magnitude below the no observable effect concentration. The ecological and human health impacts now, and the continued use of HFO-1234yf in India, China, and the Middle East, are estimated to be insignificant based on the current understanding, as summarized by Neale et al. (2021).
“Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020” (Neale et al) 2021 paper[7].
The paper includes a comprehensive summary for TFA and points out that most PFAS have different properties from TFA. Some HFCs and some HFOs breakdown produce TFA in the atmosphere. The summary has these important findings, but also covers these issues in much greater detail:
- TFA continues to be found in the environment, including in remote regions, although not at concentrations likely to have adverse toxicological consequences.
- TFA is found in the environment as a salt, with a no-observed-effect-concentration (NOEC) for aquatic species, which is typically > 10,000 μg/L. TFA is produced by the environmental degradation of several hydrofluorocarbons (HFCs) and hydrofluoro-olefins (HFOs). Analysis of 1187 samples of rainwater collected in eight locations across Germany in 2018–2019 showed median and a precipitation-weighted mean concentration of TFA of 0.210 μg/L and 0.335 μg/L, respectively.
- Other sources of TFA, besides from refrigerants and propellants that fall under the purview of the Montreal Protocol, may be more important but less understood.
- Fugitive emissions of TFA have been reported from landfills, transfer stations, and incinerators in locations where manufacturing facilities produce fluorinated chemicals.
- Current concentrations of TFA salts and related compounds in soil and surface waters do not present risks of adverse effects in aquatic and terrestrial plants and animals.
- Historical and current measurements of TFA in soil and surface-water indicate de minimis risks when compared to no-effect-concentrations (NOECs) in laboratory and field-based testing.
- Humans could be exposed to TFA via drinking water and food but there is no evidence to date of adverse effects on health.
- TFA salts are of low acute toxicity to mammals under conditions relevant to environmental exposure.
In its Summary Update 2020 for Policymakers[8], the UNEP Environmental Effects Assessment Panel has summarised these scientific conclusions for TFA (paragraph 2.3 - Air quality and contaminants):
“The current low concentration of trifluoroacetic acid (TFA) produced by the degradation of several hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), is currently judged not to pose a risk to human health or to the environment”.
“Trifluoroacetic acid continues to be found in the environment, including in remote regions, although concentrations are currently very unlikely to have adverse toxicological consequences for humans and ecosystems. While TFA is formed from the HFCs and HFOs regulated under the Montreal Protocol, a large amount of TFA was naturally formed over millions of years and has accumulated in the oceans. An unknown amount originates from fugitive emissions from chemical manufacture, waste disposal sites, laboratory use, and degradation of pharmaceuticals, pesticides, and industrial chemicals containing the trifluoromethyl group.”
References
[1] Henne, S.; Shallcross, D. E.; Reimann, S.; Xiao, P.; Brunner, D.;O’Doherty, S.; Buchmann, B., “Future emissions and atmospheric fate of HFC-1234yf from mobile air conditioners in Europe”, Environmental Science and Technology, 2012, 46, 1650−1658.
[2] Luecken, D. J.; Waterland, R. L.; Papasavva, S.; Taddonio, K. N.; Hutzell, W. T.; Rugh, J. P.; Andersen, S. O., “Ozone and TFA impacts in North America from degradation of 2,3,3,3-tetrafluoropropene (HFO-1234yf), a potential greenhouse gas replacement”, Environmental Science and Technology, 2010, 44, 343−348.
[3] Papasavva, S.; Luecken, D. J.; Waterland, R. L.; Taddonio, K. N.; Andersen, S. O., “Estimated 2017 refrigerant emissions of 2, 3, 3, 3-tetrafluoropropene (HFC-1234yf) in the United States resulting from automobile air conditioning”, Environmental Science and Technology, 2009, 43, 9252−9259.
[4] Kazil, J.; McKeen, S.; Kim, S. W.; Ahmadov, R.; Grell, G. A.; Talukdar, R. K.; Ravishankara, A. R., “Deposition and rainwater concentrations of trifluoroacetic acid in the United States from the use of HFO-1234yf”, J. Geophys. Res.-Atmos. 2014, 119, 14-059−14-079.
[5] Wang, Z.; Wang, Y.; Li, J.; Henne, S.; Zhang, B.; Hu, J.; Zhang, J., “Impacts of the Degradation of 2,3,3,3-Tetrafluoropropene into Trifluoroacetic Acid from Its Application in Automobile Air Conditioners in China, the United States, and Europe”, Environmental Science and Technology 2018, 52, 2819−2826.
[6] Liji M. David, Mary Barth, Lena Höglund-Isaksson, Pallav Purohit, Guus J. M. Velders, Sam Glaser, and A. R. Ravishankara, “Trifluoroacetic acid deposition from emissions of HFO-1234yf in India, China, and the Middle East”, Atmospheric Chemistry and Physics, 21, 14833–14849, 2021
[7] Neale, R. E., Barnes, P. W., Robson, T. M., Neale, P. J., Williamson, C. E., Zepp, R. G., et al. (2021), ““Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020”, Photochemical & Photobiological Sciences.
[8] Environmental Effects Assessment Panel Update 2020.docx (unep.org)