TFA: what is it, how much is formed from F-gases, and what are its environmental effects
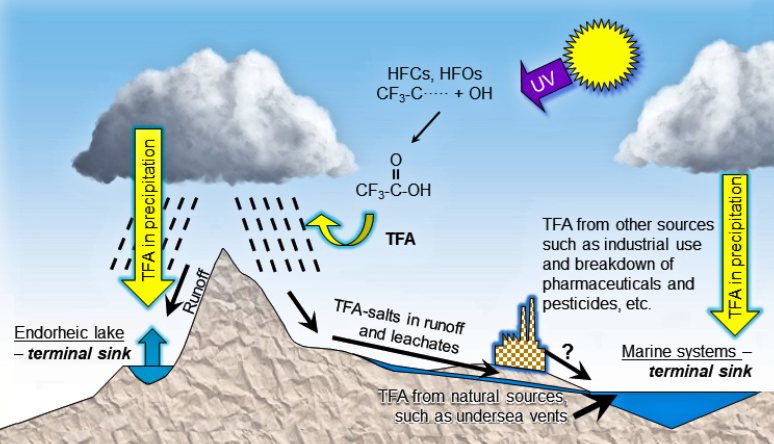
Image Reproduced from EEAP. 2019. Environmental Effects and Interactions of Stratospheric Ozone Depletion, UV Radiation, and Climate Change. 2018 Assessment Report. Nairobi: Environmental Effects Assessment Panel, United Nations Environment Programme (UNEP) 390 pp. https://ozone.unep.org/science/assessment/eeap
TFA (trifluoroacetic acid and its salts) occurs naturally in the oceans, estimated at 61 -205 million tonnes,[1] formed over millions of years. It is well established that TFA is a ubiquitous natural component in rivers, lakes, and other surface water bodies. It is found in the environment as a salt. Natural sources are thought to include undersea volcanic vents. Before 1947 extremely limited quantities (if any) of TFA were emitted from industrial sources, but it is found in the deep arctic ocean layers in waters that have an average age comparably high at perhaps 400 years.[2] Most PFAS have different properties from TFA.[3]
TFA is known to be widespread in the aquatic environment and can be introduced into the water cycle through industrial processes and as a transformation product of pharmaceutical and agricultural products among others. TFA is also a transformation product of some HFCs, HFOs and HCFOs in the atmosphere and reaches the aqueous environment via atmospheric deposition.

Transport of TFA over land by sea salt aerosol occurs, but without an enhancement mechanism removing TFA from the sea water, a simple mechanical process would only result in minor quantities of TFA deposition and cannot account for the much larger than expected rainwater concentrations in the 1990s estimated from known TFA sources at that time. If evaporation of TFA occurs within the marine boundary layer during generation of the aerosol, the quantity of TFA introduced into the atmosphere could be very much larger. TFA does have a small but significant vapour pressure over simulated sea salt aerosol and can partition into the gas phase,[4],[5] but the magnitude of this effect is unknown.[6] Volatilisation of longer chain perfluorinated carboxylic acids has been shown to occur, without aerosol formation, at concentrations 10 times lower than the TFA found in the oceans.[7]
Key points from the 2020 comprehensive independent summary of TFA effects from the UNEP Environmental Effects Assessment Panel3
- The current low concentration of TFA produced by the degradation of several HFCs and HFOs is currently judged not to pose a risk to human health or to the environment
- Humans could be exposed to TFA via drinking water and food but there is no evidence to date of adverse effects on health
- TFA salts are of low acute toxicity to mammals under conditions relevant to environmental exposure
- Other sources of TFA, besides from refrigerants and propellants that fall under the purview of the Montreal Protocol, may be more important but less understood
TFA and its salts are very water soluble
As TFA is highly water soluble, poorly absorbed and therefore very mobile, it can very rapidly enter the natural water cycle through the atmosphere, soil and wastewater. It is virtually impossible to lock TFA into the soil. TFA is stable in the environment and is persistent. Amounts deposited in flowing surface water will ultimately accumulate mainly in the oceans and lakes where water is lost only by evaporation (land locked lakes and salt lakes).[8]
Because of their high solubility in water and their very small octanol-water partition coefficient, TFA salts do not bioconcentrate in aquatic organisms, and do not biomagnify in the food chain. Thus, they present negligible risk to organisms higher on the food chain, including humans under conditions relevant to environmental exposure.[9]
Ecotoxicological data
TFA’s toxicity has been tested on various aquatic and terrestrial species and can be assessed as low. Based on the available studies, TFA is of no health concern at the measured concentrations and is not harmful to ecosystems.8
Extensive studies for TFA deposition from HFCs and HFOs
Some HFCs, HFOs and HCFOs generate TFA when they breakdown in the atmosphere. Extensive experimental studies and detailed atmospheric modelling means that the quantities of TFA generated from F-gases are well characterised and deposition patterns understood.
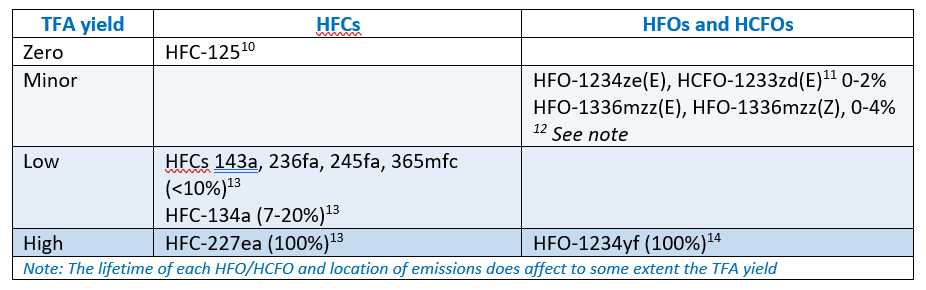
Yields of TFA from HFCs, HFOs and HCFOs that contain CF3-C grouping (excludes HFC-32 and HFC-152a)
Because HFO-1234yf breaks down to give 100% TFA, considerable work has investigated the environmental effects, if any, due to its use and emissions. It is widely used in mobile air-conditioning and studies have focussed on this use. The contribution of the other HFOs and HCFO to TFA generation from forecast EU emissions is very minor.[15],[16]
“Future emissions and atmospheric fate of HFC-1234yf from mobile air conditioners in Europe” [17]: These highest annual mean concentrations of TFA are at least 60 times lower than previously determined to be a safe level for the most sensitive aquatic life-forms. Rainwater concentrations during individual rain events would still be one order of magnitude lower than the no effect level[a].
Other similar studies from the use of HFO-1234yf for the USA and for India, China and the Middle East reached similar conclusions, finding TFA concentrations orders of magnitude below the no observable effect concentration.[18],[19]
Drinking water and TFA concentrations from HFCs and HFOs
The German Federal Environment Agency (UBA) guidance value for TFA in drinking water is based on available toxicological studies for TFA salts. This sets a drinking water health guidance value of 60 μg/L and a target value of 10 μg/L. The health guidance value is based on the life-long tolerable daily intake of TFA via the drinking water (assumption: 2 L per day), in which no harm to human health is to be expected.[20]
The study “Future emissions and atmospheric fate of HFC-1234yf from mobile air conditioners in Europe” concluded that the contribution of TFA from HFO-1234yf to European mean TFA concentrations in rainwater were estimated at 0.6 μg/L to 0.8 μg/L. Within the EU annual mean concentrations were predicted to be highest in the Czech Republic and Southern Germany (1.7 μg/L), with a European maxima of 2.2 μg/L.
Analysis of 1187 samples of rainwater collected in eight locations across Germany in 2018–2019 showed median and a precipitation-weighted mean concentration of TFA of 0.210 μg/L and 0.335 μg/L, respectively.3
Some concentrations of TFA measured in the oceans are similar to rainwater concentrations: 0.19 to 0.21 μg/L in the mid-Atlantic at 8 different depths[21] and 0.16 μg/L in the deep Arctic Ocean1 where the average age of the Canadian Basin Deep Waters is comparably high at perhaps 400 years.
Conclusion
The formation and deposition of TFA from HFCs, HFOs and HCFOs has been widely studied, and results conclude that the current low concentration of TFA produced by the degradation of several HFCs and HFOs is currently judged not to pose a risk to human health or to the environment. Extensive studies for the ecotoxicological effects have demonstrated that TFA from HFCs, HFOs and HCFOs is of no health concern at the measured concentrations and at these concentrations is not harmful to ecosystems. TFA occurs naturally in the oceans at concentrations similar to those found in rainwater.
References
[a] The no observed effect concentration (NOEC) for most aquatic species is >10 000 μg/L (see reference 14) with the most sensitive alga at 120 μg/L and Environmental Risk Assessment of Trifluoroacetic Acid 1999, Human and Ecological Risk Assessment 5(1):59-124 DOI: 10.1080/10807039991289644.
[1] B. F. Scott, R. W. Macdonald, K. Kannan, A. Fisk, A. Witter, N. Yamashita, L. Durham, C. Spencer and D. C. G. Muir, Trifluoroacetate profiles in the Arctic, Atlantic, and Pacific Oceans, Environ. Sci. Technol., 2005, 39(17), 6555–6560.
[2] The arctic ocean component in the Greenland-Scotland overflow, B Rudels and D Quadfasel, International Council for the Exploration of the Sea paper C.M. 1991/C:30.
[3] Neale, R. E., Barnes, P. W., Robson, T. M., Neale, P. J., Williamson, C. E., Zepp, R. G., et al. (2021). Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020. Photochemical & Photobiological Sciences. https://doi.org/10.1007/s43630-020-00001-x. See sections 7.8 to 7.11 for Trifluoroacetic acid (TFA).
[4] D.J. Bowden, S.L, Clegg, P. Brimblecombe, The Henry's Law Constant of Trifluoroacetic Acid and its Partitioning into Liquid Water in the Atmosphere, Chemosphere, 32(2), 405-420, doi:10.1016/j.atmosenv.2007.11.009, 1996.
[5] S. Kutsuna, and H. Hori, Experimental determination of Henry’s law constants of trifluoroacetic acid at 278–298K, Atmos. Environ. 42, 1399–1412, doi.org/10.1016/j.atmosenv.2007.11.009, 2008.
[6] EFCTC Paper, Transport of naturally occurring trifluoroacetic acid (TFA) by sea salt aerosol, 2020, available at EFCTC-Paper-Transport-of-natural-TFA-by-Sea-Salt-Aerosol-02.04.2020-1-1-1.pdf (fluorocarbons.org).
[7] Water-to-air transfer of perfluorinated carboxylates and sulfonates in a sea spray simulator, M. Reth et al., Environ. Chem. 2011, 8, 381–388. doi:10.1071/EN11007.
[8] Reducing chemical input into water bodies – trifluoroacetate (TFA) as a persistent and mobile substance from many sources, K. Adlunder et al, 2021, German Environment Agency, www.umweltbundesamt.de/en.
[9] UNEP Ozone Secretariat, Ecological Issues on the feasibility of managing HFCs: Focus on TFA Inter-sessional informal meeting, 12-13 June 2015 Informal Brief on Ecological Issues on HFCs June 2015
[10] IPCC/TEAP Special Report Safeguarding the Ozone Layer and the Global Climate System: Issues Related to Hydrofluorocarbons and Perfluorocarbons Chapter 2 page 153
[11] Mads P. Sulbaek Andersen, Johan A. Schmidt, Aleksandra Volkov, Donald J. Wuebbles, A three-dimensional model of the atmospheric chemistry of E and ZCF3CH=CHCl (HCFO-1233(zd) (E/Z)), Atmospheric Environment Volume 179, April 2018, Pages 250-259.
[12] EFCTC Position Paper: Published evidence supports very low yield of TFA from most HFOs and HCFOs available at https://www.fluorocarbons.org/publication/published-evidence-supports-very-low-yields-of-tfa-from-most-hfos-and-hcfos/
[13] World Meteorological Organisation (2010) Global Ozone Research and Monitoring Project Report No. 52. Scientific Assessment of Ozone Depletion. Table1.11 Available at: https://www.wmo.int/pages/prog/arep/gaw/ozone_2010/documents/Ozone-Assessment-2010-complete.pdf
[14] Solomon, K.R., Velders, G. J. M., Wilson S. R., Madronich S., Longstreth J., Aucamp P. J., (2016) Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols. Journal of Toxicology and Environmental Health, Part B Critical Reviews, 19, 289-304. https://doi.org/10.1080/10937404.2016.1175981
[15] UBA Final report Persistent degradation products of halogenated refrigerants and blowing agents in the environment: type, environmental concentrations, and fate with particular regard to new halogenated substitutes with low global warming potential | Umweltbundesamt.
[16] CONTRIBUTION OF HFO-1234ze, HFO-1336mzz AND HCFO-1233zd TO TFA CONCENTRATION IN EUROPEAN RAINWATER EXTREMELY SMALL - Fluorocarbons
[17] Henne, S.; Shallcross, D. E.; Reimann, S.; Xiao, P.; Brunner, D.;O’Doherty, S.; Buchmann, B., “Future emissions and atmospheric fate of HFC-1234yf from mobile air conditioners in Europe”, Environmental Science and Technology, 2012, 46, 1650−1658.
[18] Kazil, J.; McKeen, S.; Kim, S. W.; Ahmadov, R.; Grell, G. A.; Talukdar, R. K.; Ravishankara, A. R., “Deposition and rainwater concentrations of trifluoroacetic acid in the United States from the use of HFO-1234yf”, J. Geophys. Res.-Atmos. 2014, 119, 14-059−14-079.
[19] Liji M. David, Mary Barth, Lena Höglund-Isaksson, Pallav Purohit, Guus J. M. Velders, Sam Glaser, and A. R. Ravishankara, “Trifluoroacetic acid deposition from emissions of HFO-1234yf in India, China, and the Middle East”, Atmospheric Chemistry and Physics, 21, 14833–14849, 2021
[20] Trifluoressigsäure (TFA)–Gewässerschutz im Spannungsfeld von toxikologischem Leitwert, Trinkwasserhygiene und Eintragsminimierung. Erläuterungen zur Einordnung des neuen Trinkwasserleitwerts von 60 μg/L. 20. Oktober 2020. Umweltbundesamt www.umweltbundesamt.de
[21] H. Frank, E. H. Christoph, O. Holm-Hansen and J. L. Bullister, Trifluoroacetate in ocean waters, Environ.Sci. Technol., 2002, 36(1), 12–15.