Atmospheric Abundance and Emissions
For many years atmospheric monitoring stations, a network of global background air sites, have collected air samples and analysed them for a whole range of pollutants including fluorocarbons, carbon dioxide, methane, and nitrous oxide. Global averages (annual mean mole fractions) for a wide range of pollutants and their trends are calculated. Global emissions are derived from these background atmospheric measurements. The Figure from NOAA shows the concentrations and trends of well-mixed, long-lived greenhouse gases (to different scales). Further data for other individual HFC concentrations is available in the Scientific Assessment of Ozone Depletion 2022: Figure 2-1 (available at https://ozone.unep.org/science/assessment/sap)
Figure- Concentrations of some well-mixed, long-lived greenhouse gases (from NOAA)
Image is from https://gml.noaa.gov/aggi/aggi.html. Figure 2. Global average abundances of the major, well-mixed, long-lived greenhouse gases – carbon dioxide, methane, nitrous oxide, CFC-12 and CFC-11 – from the NOAA global air sampling network since the beginning of 1979. These five gases account for about 96% of the effective radiative forcing by long-lived greenhouse gases since 1750. The remaining 4% is contributed by 17 other halogenated gases including HCFC-22 and HFC-134a, for which NOAA observations are also shown here.
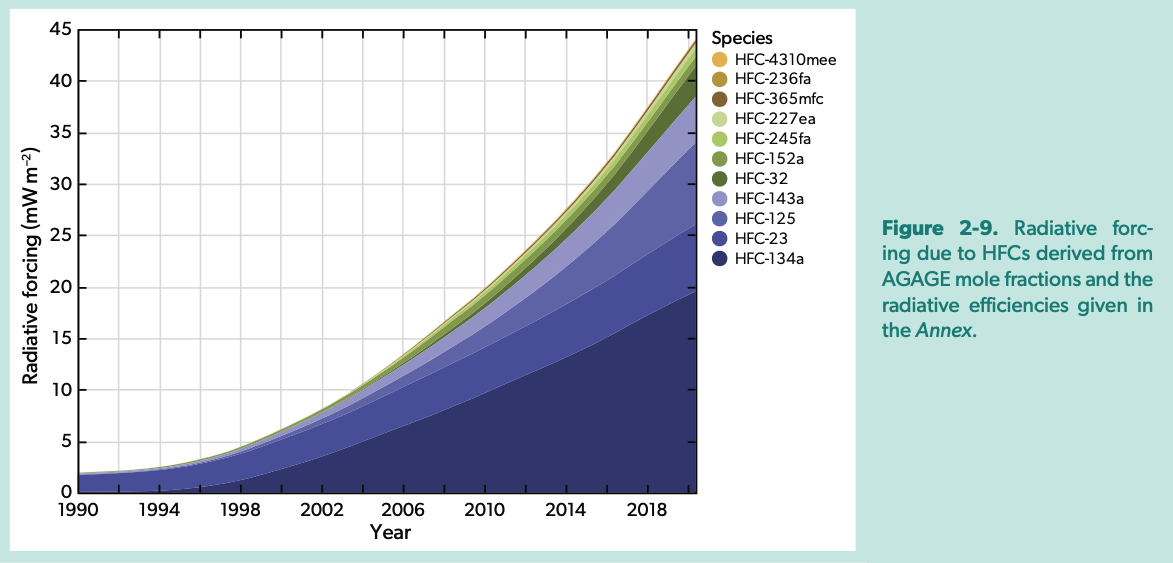
Emissions of the HFCs as CO2-equivalent using GWP-100 (from Scientific Assessment of Ozone Depletion 2022)
Compared to HFCs, the HFOs and HCFOs breakdown very quickly in the atmosphere due to their short atmospheric lifetimes measured in days or months. Now that HFOs are becoming more widely used in the EU, very low atmospheric concentrations have been detected in Central Europe. Measurements show that the background atmospheric abundances of both compounds in central Europe have continued to grow, from less than 0.01 ppt before 2016 to annual median levels of 0.10 and 0.14 ppt for HFO-1234yf and HFO-1234ze(E), respectively, in 2020. (from Scientific Assessment of Ozone Depletion: 2022). However, it is unlikely that the abundances of the HFOs measured for Central Europe are representative of global abundances due to their short atmospheric lifetimes and the early adoption of HFOs in Europe.
Monitoring fluorocarbon atmospheric concentrations and emission sources: For many years atmospheric monitoring stations around the globe have collected air samples and analysed them for a whole range of pollutants including fluorocarbons, carbon dioxide, methane, and nitrous oxide (see below). Spikes in specific pollutants can be traced to a general source area based on prevailing air flow patterns. The sources of the pollutant may be well known and could be from existing banks of fluorocarbons in equipment such as refrigerators, air-conditioners, or motor vehicles if recovery and destruction of the fluorocarbon is not undertaken and may not be linked to current fluorocarbon manufacturing. Equipment such as refrigerators can have lifetimes of over 10 years before disposal. In the case of fluorocarbons used in insulating foam, there is a slow leakage through the closed cells of the foam over a long period of time but most of the emission can occur after the foam is scrapped (if no provision is made to capture and destroy it). The atmospheric lifetimes of fluorocarbons, carbon dioxide methane, and nitrous oxide are established and these lifetimes together with their concentrations in the atmosphere enable the magnitude of any emissions to be calculated. If the substance is being destroyed in the atmosphere faster than it is being emitted then the atmospheric concentration will decrease. Conversely, significant new sources of emissions will be detected.